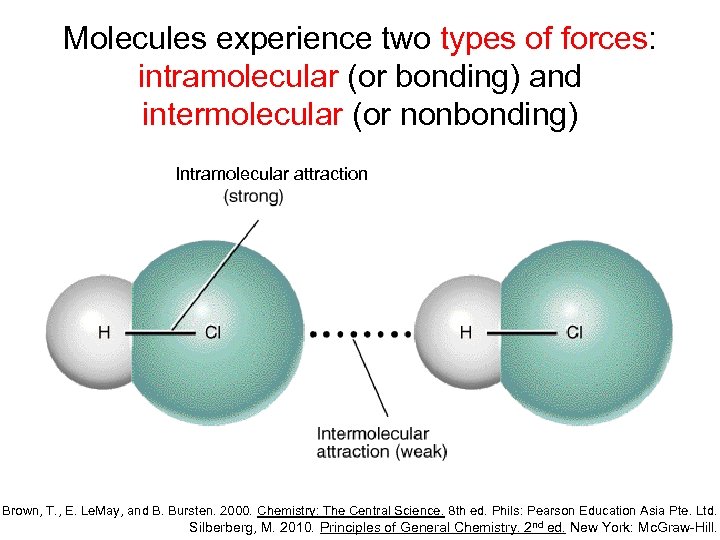
Which Property Of Matter Is Not Affected By Intermolecular Forces . Melting and boiling points of the halogens. Physical properties of liquids and solids are due to intermolecular forces. The three main types of intermolecular forces are. The comprehension of intermolecular forces helps us to understand and explain the physical properties of substances, since it is intermolecular forces that. Learn about intermolecular forces, the ideal gas law,. Under appropriate conditions, the attractions between all gas molecules will cause them to form liquids or. Intermolecular forces are much weaker than the intramolecular forces of attraction but are important because they determine the physical. This unit explores the factors that determine the physical properties of matter. Intermolecular forces are attractive and repulsive forces between atoms, groups of atoms, or ions in separate molecules. The increase in melting and boiling points with increasing atomic/molecular size. Depending on its strength, intermolecular forces cause the forming of three physical states: These are forces between molecules.
from present5.com
Learn about intermolecular forces, the ideal gas law,. Physical properties of liquids and solids are due to intermolecular forces. Melting and boiling points of the halogens. The comprehension of intermolecular forces helps us to understand and explain the physical properties of substances, since it is intermolecular forces that. The increase in melting and boiling points with increasing atomic/molecular size. Intermolecular forces are attractive and repulsive forces between atoms, groups of atoms, or ions in separate molecules. Intermolecular forces are much weaker than the intramolecular forces of attraction but are important because they determine the physical. The three main types of intermolecular forces are. This unit explores the factors that determine the physical properties of matter. Depending on its strength, intermolecular forces cause the forming of three physical states:
Forces at work in a molecule Molecules
Which Property Of Matter Is Not Affected By Intermolecular Forces Intermolecular forces are much weaker than the intramolecular forces of attraction but are important because they determine the physical. Physical properties of liquids and solids are due to intermolecular forces. Learn about intermolecular forces, the ideal gas law,. This unit explores the factors that determine the physical properties of matter. Melting and boiling points of the halogens. Intermolecular forces are much weaker than the intramolecular forces of attraction but are important because they determine the physical. The increase in melting and boiling points with increasing atomic/molecular size. Intermolecular forces are attractive and repulsive forces between atoms, groups of atoms, or ions in separate molecules. These are forces between molecules. The three main types of intermolecular forces are. The comprehension of intermolecular forces helps us to understand and explain the physical properties of substances, since it is intermolecular forces that. Depending on its strength, intermolecular forces cause the forming of three physical states: Under appropriate conditions, the attractions between all gas molecules will cause them to form liquids or.
From present5.com
Forces at work in a molecule Molecules Which Property Of Matter Is Not Affected By Intermolecular Forces Intermolecular forces are attractive and repulsive forces between atoms, groups of atoms, or ions in separate molecules. Physical properties of liquids and solids are due to intermolecular forces. Depending on its strength, intermolecular forces cause the forming of three physical states: This unit explores the factors that determine the physical properties of matter. The three main types of intermolecular forces. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of Which Property Of Matter Is Not Affected By Intermolecular Forces This unit explores the factors that determine the physical properties of matter. The comprehension of intermolecular forces helps us to understand and explain the physical properties of substances, since it is intermolecular forces that. The increase in melting and boiling points with increasing atomic/molecular size. Intermolecular forces are attractive and repulsive forces between atoms, groups of atoms, or ions in. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From sciencenotes.org
Intermolecular Forces in Chemistry Which Property Of Matter Is Not Affected By Intermolecular Forces Melting and boiling points of the halogens. Learn about intermolecular forces, the ideal gas law,. This unit explores the factors that determine the physical properties of matter. Intermolecular forces are attractive and repulsive forces between atoms, groups of atoms, or ions in separate molecules. Physical properties of liquids and solids are due to intermolecular forces. These are forces between molecules.. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint Which Property Of Matter Is Not Affected By Intermolecular Forces The three main types of intermolecular forces are. These are forces between molecules. The increase in melting and boiling points with increasing atomic/molecular size. Melting and boiling points of the halogens. The comprehension of intermolecular forces helps us to understand and explain the physical properties of substances, since it is intermolecular forces that. Intermolecular forces are much weaker than the. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Which Property Of Matter Is Not Affected By Intermolecular Forces These are forces between molecules. Under appropriate conditions, the attractions between all gas molecules will cause them to form liquids or. Melting and boiling points of the halogens. Learn about intermolecular forces, the ideal gas law,. The three main types of intermolecular forces are. Depending on its strength, intermolecular forces cause the forming of three physical states: This unit explores. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Which Property Of Matter Is Not Affected By Intermolecular Forces The comprehension of intermolecular forces helps us to understand and explain the physical properties of substances, since it is intermolecular forces that. Depending on its strength, intermolecular forces cause the forming of three physical states: These are forces between molecules. Under appropriate conditions, the attractions between all gas molecules will cause them to form liquids or. Melting and boiling points. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From www.slideserve.com
PPT Summary of the three States of Matter ALSO CALLED PHASES, HAPPENS Which Property Of Matter Is Not Affected By Intermolecular Forces Melting and boiling points of the halogens. The increase in melting and boiling points with increasing atomic/molecular size. Intermolecular forces are attractive and repulsive forces between atoms, groups of atoms, or ions in separate molecules. Learn about intermolecular forces, the ideal gas law,. Intermolecular forces are much weaker than the intramolecular forces of attraction but are important because they determine. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint Which Property Of Matter Is Not Affected By Intermolecular Forces The three main types of intermolecular forces are. The increase in melting and boiling points with increasing atomic/molecular size. Intermolecular forces are much weaker than the intramolecular forces of attraction but are important because they determine the physical. The comprehension of intermolecular forces helps us to understand and explain the physical properties of substances, since it is intermolecular forces that.. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces Phases of Matter & Colligative Properties Which Property Of Matter Is Not Affected By Intermolecular Forces These are forces between molecules. This unit explores the factors that determine the physical properties of matter. Physical properties of liquids and solids are due to intermolecular forces. The comprehension of intermolecular forces helps us to understand and explain the physical properties of substances, since it is intermolecular forces that. The three main types of intermolecular forces are. Intermolecular forces. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From dokumen.tips
(PPT) Intermolecular Forces and Properties of Matter DOKUMEN.TIPS Which Property Of Matter Is Not Affected By Intermolecular Forces Under appropriate conditions, the attractions between all gas molecules will cause them to form liquids or. Physical properties of liquids and solids are due to intermolecular forces. The increase in melting and boiling points with increasing atomic/molecular size. The three main types of intermolecular forces are. This unit explores the factors that determine the physical properties of matter. These are. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From studylib.net
Intermolecular Forces Which Property Of Matter Is Not Affected By Intermolecular Forces These are forces between molecules. Intermolecular forces are much weaker than the intramolecular forces of attraction but are important because they determine the physical. Physical properties of liquids and solids are due to intermolecular forces. Depending on its strength, intermolecular forces cause the forming of three physical states: The three main types of intermolecular forces are. Under appropriate conditions, the. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation ID542353 Which Property Of Matter Is Not Affected By Intermolecular Forces Depending on its strength, intermolecular forces cause the forming of three physical states: The comprehension of intermolecular forces helps us to understand and explain the physical properties of substances, since it is intermolecular forces that. The increase in melting and boiling points with increasing atomic/molecular size. Intermolecular forces are much weaker than the intramolecular forces of attraction but are important. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From www.slideserve.com
PPT States of Matter and Intermolecular Forces PowerPoint Which Property Of Matter Is Not Affected By Intermolecular Forces The increase in melting and boiling points with increasing atomic/molecular size. Physical properties of liquids and solids are due to intermolecular forces. Intermolecular forces are much weaker than the intramolecular forces of attraction but are important because they determine the physical. This unit explores the factors that determine the physical properties of matter. Intermolecular forces are attractive and repulsive forces. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From quizlet.com
Intermolecular forces AP Chemistry Diagram Quizlet Which Property Of Matter Is Not Affected By Intermolecular Forces Depending on its strength, intermolecular forces cause the forming of three physical states: These are forces between molecules. Under appropriate conditions, the attractions between all gas molecules will cause them to form liquids or. The comprehension of intermolecular forces helps us to understand and explain the physical properties of substances, since it is intermolecular forces that. The increase in melting. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces; Explaining Liquid Properties PowerPoint Which Property Of Matter Is Not Affected By Intermolecular Forces This unit explores the factors that determine the physical properties of matter. Learn about intermolecular forces, the ideal gas law,. Under appropriate conditions, the attractions between all gas molecules will cause them to form liquids or. The three main types of intermolecular forces are. The increase in melting and boiling points with increasing atomic/molecular size. Intermolecular forces are much weaker. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From slidetodoc.com
Intermolecular Forces 1 States of Matter What do Which Property Of Matter Is Not Affected By Intermolecular Forces Intermolecular forces are much weaker than the intramolecular forces of attraction but are important because they determine the physical. The increase in melting and boiling points with increasing atomic/molecular size. Melting and boiling points of the halogens. Physical properties of liquids and solids are due to intermolecular forces. These are forces between molecules. Depending on its strength, intermolecular forces cause. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From www.online-sciences.com
The properties of the molecules of the matter Science online Which Property Of Matter Is Not Affected By Intermolecular Forces Under appropriate conditions, the attractions between all gas molecules will cause them to form liquids or. Depending on its strength, intermolecular forces cause the forming of three physical states: The comprehension of intermolecular forces helps us to understand and explain the physical properties of substances, since it is intermolecular forces that. The increase in melting and boiling points with increasing. Which Property Of Matter Is Not Affected By Intermolecular Forces.
From www.pearson.com
Intermolecular Forces and Physical Properties Example 1 Channels for Which Property Of Matter Is Not Affected By Intermolecular Forces Under appropriate conditions, the attractions between all gas molecules will cause them to form liquids or. These are forces between molecules. Intermolecular forces are attractive and repulsive forces between atoms, groups of atoms, or ions in separate molecules. Physical properties of liquids and solids are due to intermolecular forces. The comprehension of intermolecular forces helps us to understand and explain. Which Property Of Matter Is Not Affected By Intermolecular Forces.